Researchers at the University of Dundee have revealed in the greatest detail yet the workings of molecules called ‘protein degraders’ which can be deployed to combat what have previously been regarded as ‘undruggable’ diseases, including cancers and neurodegenerative diseases.
Protein degrader molecules are heralding a revolution in drug discovery, with more than 50 drugs of this type currently being tested in clinical trials for patients with diseases for which no other options exist.
The Centre for Targeted Protein Degradation (CeTPD) at the University of Dundee is one of the world’s leading centres for research into how protein degraders work and how they can most effectively be put to use for a new generation of drugs.
Now researchers have revealed previously invisible levels of detail and understanding of how the protein degraders work, which in turn is allowing for even more targeted use of them at the molecular level.
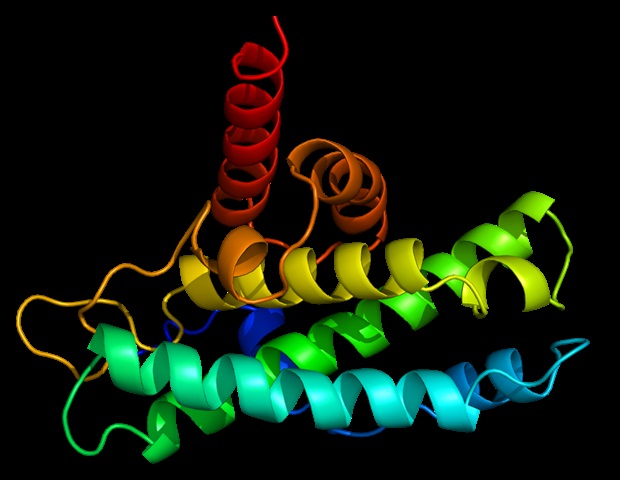
PhD student Charlotte Crowe, together with Dr Mark Nakasone, Senior Postdoctoral Scientist at CeTPD, used a technique called cryo-electron microscopy (cryo-EM), which enables scientists to see how biomolecules move and interact with each other.
This works by flash-freezing proteins and using a focused electron beam and a high-resolution camera to generate millions of 2D images of the protein. They then used sophisticated software and artificial intelligence (AI) models which allowed them to generate 3D snapshots of the degrader drugs working in action.
Their latest research is published in the prestigious journal Science Advances and is expected to constitute a landmark contribution to research in the field of TPD and ubiquitin mechanisms.
“We have reached a level of detail where we can see how these protein degraders work and can be deployed [to recruit the disease-causing protein ] and target the ‘bull’s eye’, in molecular terms,” said Charlotte Crowe, who carried out the research together with a wider team of Dundee researchers.
“Protein degrader molecules work in a way that is fundamentally different from the way conventional drugs work. However, until recently the exact details of how this process works at the molecular level had remained elusive.
“Proteins are typically a few nanometres large, which is 1 billionth of a metre, or 1 millionth of the width of a hair. So being able to ‘see’ them in action has not been possible, up until now.
“We have now been able to build a moving image of how it all happens, which means we can more specifically control the process with an incredible level of detail.“
Professor Alessio Ciulli, Director of CeTPD and one of the world leaders in the field of targeted protein degradation, said, “This is incredibly exciting work and opens up the possibility of even more effectively targeted drugs able to finally treat some diseases which up until now have been too difficult to tackle.”
How it works
Proteins are essential for our cells to function properly, but when these do not work correctly they can cause disease.
Targeted protein degradation involves redirecting protein recycling systems in our cells to destroy the disease-causing proteins.
Protein degraders work by capturing the disease-causing protein and making it stick like a glue to the cellular protein-recycling machinery, which then tags the protein as expired in order to destroy it.
The tag is a small protein called ubiquitin, which effectively gets fired at the disease-causing protein like a bullet. In order for the process to work effectively, ubiquitin must hit the right spots on the target protein so that it gets tagged effectively. The new work by the Dundee team enables them to see how the bullet hits the proverbial bull’s eye.
Working with a protein degrader molecule called MZ1, which was developed in the Ciulli laboratory at Dundee, and using high-end mass spectrometry, they were able to identify exactly where on the target protein the vital ‘tags’ are added.
The work shows how degrader drugs hold onto and position disease-causing proteins, making them good targets for receiving ubiquitin molecules (ie. “ubiquitin-atable”) which then leads to their destruction inside the cell.
Protein degradation efficiency and productivity is dependent on the degrader molecule’s ability to hold tight onto the disease-causing protein, and in a position where it can most effectively act. This latest research paints a bull’s eye and holds it steady enough for the molecule to be accurately targeted.
Professor Ciulli said this and other recently published papers were contributing to rapid development of an exciting field of science and drug discovery.
“This rapidly expanding field is fascinating and complementary articles on how this cellular protein-recycling machinery works to fire ubiquitin molecules at target proteins were recently published by the laboratories of biochemists Brenda Schulman (Max-Planck Institute of Biochemistry) and Gary Kleiger (University of Nevada, Las Vegas).
“Our collective work provides a leap forward in understanding that will accelerate development of new TPD drugs in future“.
The Dundee team
This work comes from a ‘local’ collaboration between two groups of scientists at the University of Dundee.
In the Centre for Targeted Protein Degradation, led by Professor Alessio Ciulli, experts in TPD, were Charlotte Crowe, Mark Nakasone, Conner Craigon, Gajanan Sathe and Nikolai Makukhin.
They worked with Professor Ron Hay, an expert in ubiquitin, based in the School of Life Sciences, and colleagues Sarah Chandler and Mike Tatham.
Source:
Journal reference:
Crowe, C., et al. (2024). Mechanism of degrader-targeted protein ubiquitinability. Science Advances. doi.org/10.1126/sciadv.ado6492.