In a recent study published in the journal npj Digital Medicine, researchers performed a scoping review to analyze artificial intelligence (AI)-based models for type 2 diabetes mellitus (T2DM) prediction.
 Review: A scoping review of artificial intelligence-based methods for diabetes risk prediction. Image Credit: Created with the assistance of DALL·E 3
Review: A scoping review of artificial intelligence-based methods for diabetes risk prediction. Image Credit: Created with the assistance of DALL·E 3
The Growing Importance of AI in Healthcare
AI is assisting in developing prediction models for diabetes, a condition that is increasing in prevalence across the globe. Based on risk profiles, this technique tries to evaluate an individual’s risk of developing T2DM and related complications. AI allows for the identification of high-risk patients and the development of individualized preventative methods and focused therapies.
Key Stages in Developing AI Models
AI models must be developed in stages, including model creation, assessment, and translation to clinical decision support. Internal or external validation can be used, with external validation being recommended for a more complete review of the model’s generalizability. AI-powered models have emerged as a viable approach for generating prediction models for T2DM, allowing for individualized disease-preventative strategies.
Study Methodology
In the present comprehensive review, researchers explored the applications of artificial intelligence-based predictive approaches in diabetes risk prediction.
Data were searched systematically in the Scopus, PubMed, Google Scholar, and IEEE-Xplore databases for relevant longitudinal studies using artificial intelligence-based models for human subjects and published between 1 January 2000 and 19 September 2022. In addition, references to the included studies were screened to identify additional records.
Only peer-reviewed studies, original research, and conference proceedings using medical information, including electronic health records (EHRs), imaging, and multiomics, were included. The team excluded reviews, commentaries, editorials, letters, preprints, cross-sectional studies, those without AI use, those conducted on non-human subjects, those including type 1 and gestational diabetes, and those including individuals with diabetes-associated complications.
Data extracted included the titles, publication year, first author’s name, publication type, country, study’s objective, sample population, study design, participants’ demographics, methods used to ascertain T2DM diagnoses, follow-up period, and the data source type. In addition, the team recorded the number and type of modalities used, AI type, specific algorithms, and validation approach. One reviewer performed a data search, and two independently selected studies and extracted data, and discrepancies were resolved by discussing or consulting a third reviewer. A narrative synthesis approach was used for analysis.
What the Data Reveals
Initially, 1105 records were identified, of which 853 underwent title- and abstract screening, 64 underwent full-text screening, and 40 were considered for the final analysis. Most of the studies were published in the previous four years. Sample sizes ranged from 244 to 1,893,901 individuals, with diverse populations, including those from China, Finland, California, and Kuwait.
Most Common Data Types and Algorithms
Most studies were of the retrospective cohort type, analyzing data from large private datasets and publicly accessible databases such as the Canadian Primary Care Sentinel Surveillance Network (CPCSSN), the San Antonio Heart Study (SAHS), and the Tehran Lipid and Glucose Study (TLGS). Fasting blood glucose levels of 126 mg/dL or higher and glycated hemoglobin (HbA1c) of 6.5% and higher were the most commonly used criteria for ascertaining a T2D diagnosis.
Most studies used unimodal (n=30) artificial intelligence models, with only 10 using multimodal approaches. While unimodal models showed an area under the curve (AUC) value of 0.8, multimodal models were superior (AUC, 0.9). Classical machine learning (ML) models were used in most (n=10) studies, with EHRs (including sociodemographic data, family history of diabetes, lifestyle factors, anthropometric measurements, glycemic traits, serum lipid, cholesterol, and TG levels) as the most commonly used data modality.
In addition, multi-omics (such as single nucleotide polymorphisms (SNPs), metabolomic measurements, and microbiota data) were predominant, whereas medical imaging was the least utilized. Classical machine learning models used decision trees (DT) with their variations, such as quick, unbiased, efficient statistical trees (QUEST) and classification and regression trees (CART). Linear regression modeling, random forest (RF) classifiers, support vector machines (SVM), Naïve Bays (NB) classifiers, extreme gradient boosting (XGBoost), and KNN were used in 10 studies, nine studies, eight studies, five studies, four studies, and four studies, respectively.
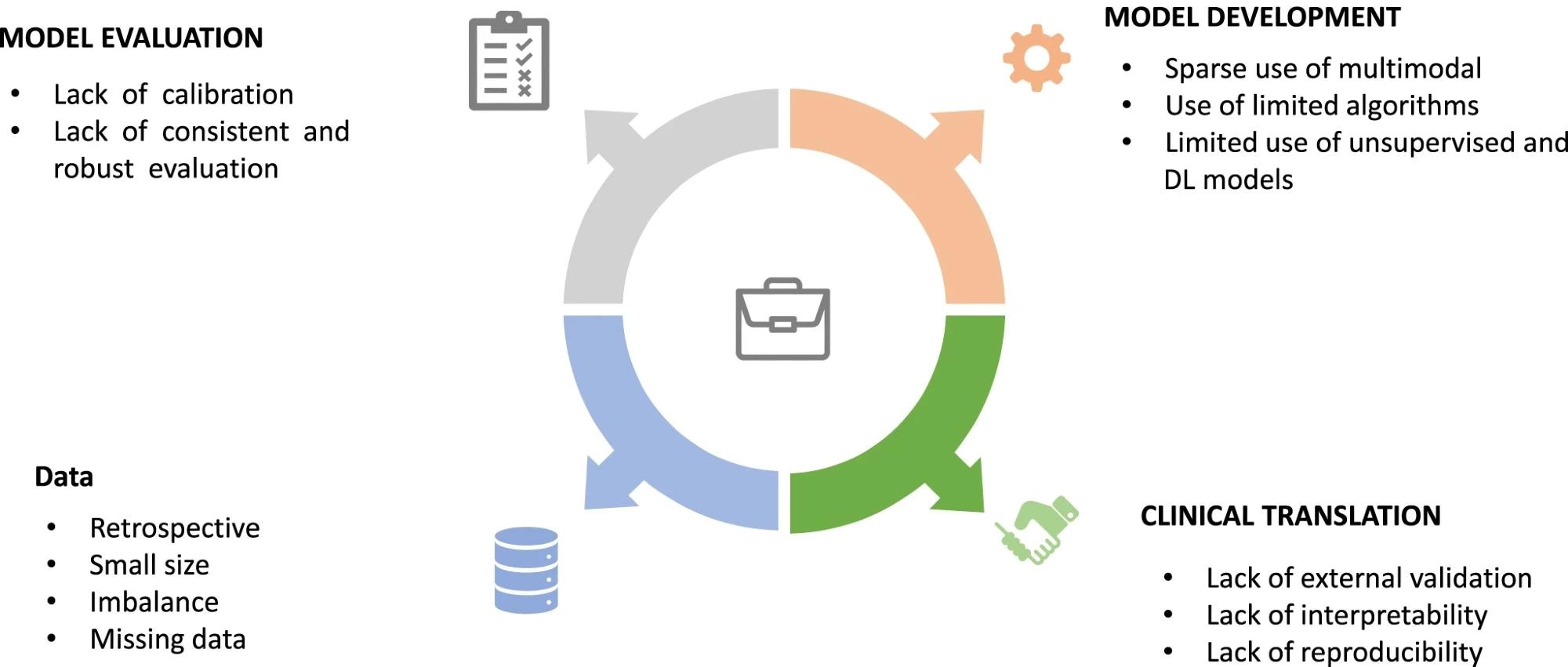 The limitations encountered at different stages of AI predictive model construction for T2DM: those associated with the underlying data, the model building and evaluation, and clinical translation.
The limitations encountered at different stages of AI predictive model construction for T2DM: those associated with the underlying data, the model building and evaluation, and clinical translation.
Validation and Interpretability: A Critical Aspect
Thirty-nine studies performed internal validation, whereas only five conducted external validation. Most studies used area under the curve (AUC) values for discrimination measures. Of note, only five studies provided model calibrations. Fifty percent of studies utilized interpretability methods to identify the risk predictors, and most models reported commonly known ones. Fasting blood glucose, body mass index (BMI), age, and serum triglyceride (TG) were the most frequently documented T2D risk predictors. Metabolomic markers included α-tocopherol, mannose, glucose, mestranol, iboflavin, hydroxysphingomyeline C14:1, and phosphatidylcholine acyl-alkyl C40:5. Imaging-based biomarkers for diabetes-related retinal disease included vascular tortuosity, retinal hemorrhage, cotton wool spots, and venous dilatation.
Future Directions and Challenges
Based on the findings, AI models have shown promise in forecasting T2DM development, but hurdles must be overcome before their full potential can be realized. To evaluate the potential advantages of AI models, extensive validation and assessment via clinical trials and prospective research are required. The function of AI in medicine is not autonomous but rather a collaborative effort between AI models and human knowledge.
Next Steps
Despite limits and hurdles, researchers must use AI technologies to speed the discovery of advancements and their translation into clinical practice for patients and healthcare professionals.




_1b78fec41a5143afb6e7b9ad317023d2-620x480.jpg)


