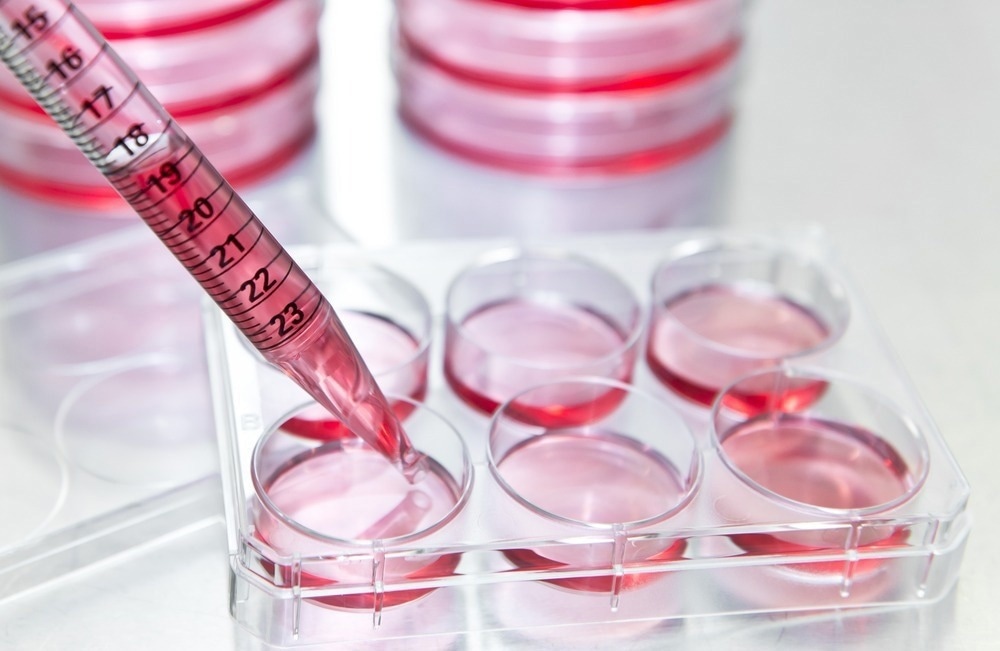
In this interview, we speak with the University of Warwick spin-out Cryologyx about their innovative new technology that hopes to revolutionize cell-based assays for researchers across life sciences and drug discovery.
Please could you introduce yourself and your role at Cryologyx?
I am Dr. Tom Congdon, the CEO and Co-Founder of CryoLogyx. Before I started CryoLogyx with Prof. Matt Gibson, I was a postdoctoral researcher at the University of Warwick.
My background is in polymer chemistry and synthesis, with a focus on developing polymer-based cryoprotectants that enable new cryopreservation outcomes in cell biology.
Who is Cryologyx, and how did it come to form?
CryoLogyx is a biotech tools company and a spin-out from the University of Warwick. We develop and provide assay-ready cell models in plated, adherent formats for use in drug discovery and other cell-based assay applications.
The company came from two specific ideas. The technology we developed was based on years of research into how synthetic polymers could mimic the form and function of antifreeze proteins. Antifreeze proteins are a fascinating class of biomolecules and evolutionary biology, made more interesting by the fact that they evolved independently in the north and south polar regions.
We developed polymers that attempted to mimic the structure and function of these antifreeze proteins. We developed a polymer that dramatically improved cell recovery for adherent cells in a monolayer format.
The other idea that CryoLogyx is founded on is the question of where we could add genuine, transformational value to the life science sector and beyond. Cell-based assays enable a whole new dimension of R&D.
We believe that our technology could be developed to make those cell-based assays as easy, simple and fast as possible, accelerate biotech research, and let anyone – engineers, physicists, and material scientists access biological models.
How important are cell assays to the drug discovery and life sciences community?
All early-stage biotech research and drug discovery utilize cell-based assays. In the drug discovery pipeline, cell-based assays are used immediately to screen potential drug candidates for function and toxicity.
After this initial stage, where millions of candidates are screened, several are selected for more functional, tailored cell-based assays before being tested in vivo. There is a push to replace in vivo testing as much as possible with advanced cell-based models to reduce the number of animals needed to develop new treatments and therapies.
So, cell-based assays are vital for modern drug discovery and will only become more critical in the future.
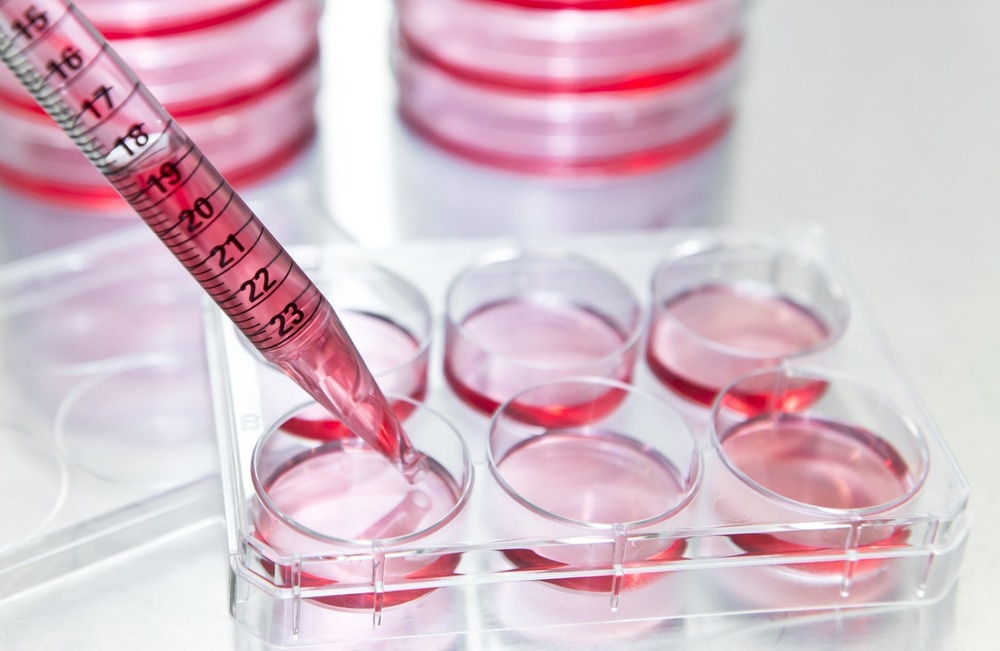
Image Credit: unoL/Shutterstock.com
What are the challenges that limit the efficiency of cell assays?
The difficulties come from maintaining cell culture. Cells are generally quite simple to culture, but the process takes time. During this time, cells will multiply and subtly change (genetic drift), which needs to be monitored.
Cells can easily cross-contaminate, mutate beyond their original makeup, and become infected, even in the most rigorous of setups. It is estimated that 15 – 20% of cell lines currently in use may not be what they are documented and reported to be.
As recently as 2008, 40 human thyroid cancer cell lines were analyzed by genetic profiling. Only 23 unique profiles were obtained, and many cross-contaminating cell lines were not even thyroid in origin.
These cell lines had been previously used for two decades in the field of thyroid cancer research.1
As a result, maintaining cultures becomes difficult, time-consuming, and expensive. Growing enough cells to meet a large screening target is as much an art as a science. A large screen will usually involve lots of moving parts, and if equipment or the candidates are delayed, cells are usually discarded, and the whole process starts again.
For smaller operations, skills and contamination are the major issues. Researchers with experience in specific cell models are in short supply, and labs can only culture so many different cell lines at one time. These limit capability and capacity, ultimately slowing down scientific progress.
Could you discuss your cell culture solutions and how they came to be developed?
As we are developing a frozen pre-plated ‘platform’ that can be applied to many different cell lines and formats, we wanted to focus on where we could create products that would have the broadest need and provide the most value to researchers.
We looked at routine cell culture operations that would benefit from being fast and simple to carry out, and earlier this year carried out an alpha test, delivering plates to cell users all over the UK.
From that, we found that users working with viruses engaged with the plates, as they wanted to focus their efforts on studying the virus, not the cells. From users in drug discovery, we found that they wanted hepatocytes in high-density formats for screening applications and assay-ready transwell plates, as these assays generally require a month of preparatory cell culture before they can be used!
What are some of the case studies where these plates have been utilized?
Virologists have used our plates in plaque assays and viral neutralization assays. We have also carried out drug toxicity screening using our assay-ready plates, measuring dose responses comparable to conventionally cultured cells.
Cryologyx is working on a range of plates for drug discovery experiments, due for release in 2023, and is now signing up researchers for pre-release evaluations.
Image Credit: unoL/Shutterstock.com
A key component of Cryologyx’s pre-plated solutions is that cell culture processes are eliminated. What benefit would this bring to a researcher’s workflow and, ultimately, the drug discovery process?
For the researcher, the primary benefit is time-saving and time to experiment. Experiments can be carried out on demand with no preparatory cell culture required and workflows shortened.
Cutting out cell culture saves around 5 – 6 hours of hands-on work per experiment. In addition, a cell-based assay can be carried out in around a day from inception, compared to currently 1 – 2 weeks from inception.
As plates can be stored frozen and made ready to use in 24 hours, workflows are far more flexible. There are also significant advantages to reproducibility, as a large bank of cells can be prepared, with the same passage number, meaning that there would be no batch-to-batch variation over months of testing.
Ultimately, drug discovery becomes faster, more flexible, and more reliable.
Cryologyx’s pre-plated cells use significantly less amount of plastic waste compared to existing products. Why is it so important for the life sciences community to try and incorporate more sustainable approaches to research, especially in routine experiments?
A study in Nature estimated that for institutes and SMEs, each bench scientist generates around a ton of plastic waste per year. It was estimated in 2014 that around 5.5 million tons of lab plastic waste were generated worldwide, equivalent to the combined tonnage of 67 cruise liners and equal to 83% of the plastic recycled worldwide in 2012.
Such an amount of single-use plastic generated is unsustainable, not only from an environmental and sustainability angle but a financial angle too.
Single-use plastic is cheap, but with costs rising for plastic and energy, it will not be forever. Businesses and Universities are looking to reduce their plastic waste and life science laboratories will soon be under the microscope.
There is already some fantastic equipment and initiatives to recycle and reduce plastic waste in the lab, such as My Green Lab and Grenova’s TipNovus machine.
At scale, we can make large batches of plates that require far less plastic to be used. We are also looking at recyclable plate ideas. For smaller labs, using our pre-plated cells would enable around an 86% reduction in single-use plastic waste generated to run five plates of experiments compared to conventional cell culture work.
You recently attended Lab Innovations; what benefits can these events bring to SMEs, especially those that are university spin-outs?
Nothing beats face-to-face interaction with customers and clients. For start-ups and spin-outs developing their business model, feedback and opportunities are vital to helping shape understanding and develop relationships with your first customers.
Attending shows is also the best way to develop a brand presence and legitimize your company in your customer’s eyes. Finally, for SMEs with our headcount, mostly focussed on R&D, getting the team out of the lab and meeting end users was a fantastic experience.
Do you have any highlights from the event?
It was great to meet so many people in person and network with the major players in lab automation and robotics. We got to meet researchers and lab managers who will be building new labs and expanding their research operations, so it was great to show them the plates and discuss the potential of assay-ready plates for these new facilities.
What are the next steps for Cryologyx?
Over the summer, we tested our products with early adopters. Based on that feedback, we launched a range of cryopreserved pre-plated assay-ready products for use in viral detection and analysis.
Now Cryologyx is working on a product range for early-stage drug discovery and toxicity testing. We are expanding our capability and, next year, will build an automated cell culture manufacturing facility to produce large volumes of assay-ready plates. We will also work with our partners in pharma and CROs to expand and accelerate and simplify their cell assay operations.
Where can our readers go to stay up to date with the company’s activities?
We regularly post news and information on our website, www.cryologyx.com, and our LinkedIn page.
You can find our two most recent publications here:
- Murray, K., Kinney, N.L.H., Griffiths, C.A., Hasan, M., Gibson, M.I., Whale, T, Scientific Reports, 2022, 12, 12295 Pollen derived macromolecules serve as a new class of ice-nucleating cryoprotectants
- Tomas, R.F.M., Bissoyi, A., Congdon, T.R., Gibson, M.I., Biomacromolecules, 2022 23, 3948-3959. Assay-ready Cryopreserved Cell Monolayers Enabled by Macromolecular Cryoprotectants,
About Dr. Tom Congdon
 Tom, the CEO of CryoLogyx, has been at the forefront of macromolecular cryoprotectant research for the last 8 years and has developed several cryoprotectant technologies.
Tom, the CEO of CryoLogyx, has been at the forefront of macromolecular cryoprotectant research for the last 8 years and has developed several cryoprotectant technologies.
Tom has a Ph.D. in the chemistry and synthesis of Cryoprotectant Materials, and his postdoc research focussed on polymer and bioconjugate synthesis and the evaluation of new cryoprotectant technologies.
In the Summer of 2020, Tom successfully completed the ICURe (Innovation to Commercialisation of University Research) program and subsequently founded CryoLogyx, which was awarded £300k from Innovate UK to begin commercial development of never seen frozen cell products. After completing the prestigious ICURe customer discovery program and securing an Innovate grant and SEIS funding from Angel investors and Oxford Technology, Tom founded CryoLogyx with Professor Matthew I Gibson.
References
- https://www.sigmaaldrich.com/GB/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/cell-culture-troubleshooting-cell-line-misidentification
- https://www.nature.com/articles/528479c