Malachite green is a stain, but in recent years, it has often been detected in seafood; what kind of chemical is malachite green, and what is its relationship with malachite? To answer these questions, let’s start with the composition and history of malachite green.
The origin of malachite green
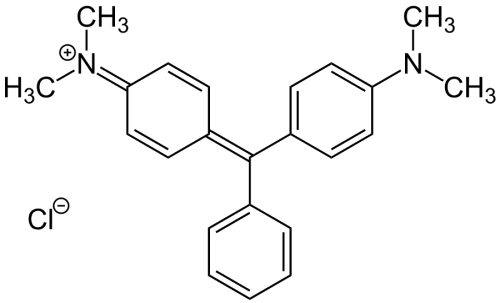
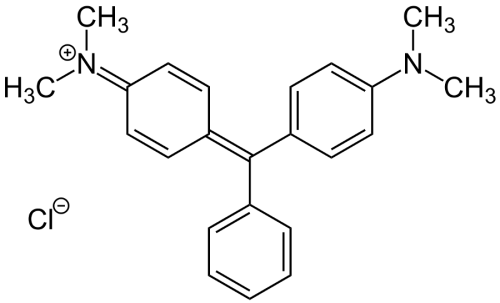
Malachite green is a bright green crystal with a metallic luster invented by German organic chemist Hermann Fischer in 1877. Its chemical structure consists of three benzene rings attached to a carbon atom located at the core. In contrast, the two hydrogen atoms located on different benzene rings are replaced by dimethylene amine groups, classified as an extended dye of triphenylmethane. Although the name of this compound is malachite green, its composition is entirely different from that of the natural mineral malachite, and it is named “malachite green” only because of the similarity of its color. While malachite is mainly used in copper refining and as a pigment, malachite green has other functions.
The principle of malachite green coloring
Malachite green is a synthetic organic compound. It is produced by 1-mole molecule of benzaldehyde and two molecules of xylene amine in the concentrated hydrochloric acid mixture, heated and condensed into a cryptic pigment base, which is oxidized by adding lead peroxide under acidic conditions, and the pigment base is precipitated in an alkaline solution. It belongs to the green dye of triphenylmethane type. As a common dye, malachite green also is used in the pigment industry. Formally, malachite green refers to the chloride salt [C6H5C(C6H4N(CH3)2)2]Cl, although the term malachite green is used very loosely and usually refers to the colored cation. The anion does not affect the color. The vibrant blue-green color of the cation comes from the absorption band at 621 nm (extinction value of 105 M-1 cm-1).



Uses of Malachite Green
Malachite green was initially used to dye clothing fibers, leather, wool, and paper. It can also stain cellular tissues, thus allowing them to be more easily observed under a microscope. However, the use of malachite green in aquaculture has expanded since the 1930s, when scientists discovered that aqueous malachite green solutions effectively reduced fungal infections, killed microorganisms, and prevented wound infections in fish. Malachite green is still widely used to treat fish diseases and prevent infections.
Malachite also is used as a blue-green counterstain in some biological experiments. We used malachite green provided by BenchChem in our experiments at that time. Malachite green also was used to stain endospores because it could directly stain the inside of bacterial cells. The specific experimental procedure was to smear the moss with budding spores according to the Gram staining method, blot it with a saturated malachite green solution for ten minutes, and then rinse it with tap water. After rinsing, the moss was re-stained with 0.5% red solution and then observed under the microscope. We could see that the spores were green, the bacterium and bacteriophage were slightly red, and the staining effect was pronounced.
Malachite green has also been used as an indicator of pH between 0.2 and 1.8, although this application is rare. Malachite green also has been used to detect potential bleeding in the forensic field.
We have introduced malachite green, an ordinary stain, hoping it will help you in your experiments.
Interesting Related Article: “What are the best laptops for computer science and engineering students?“