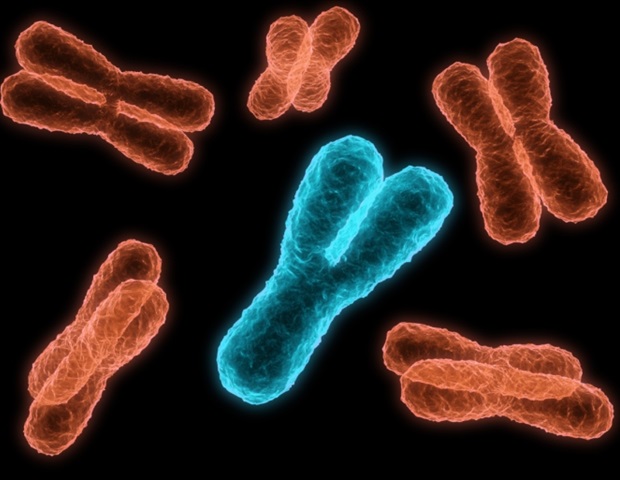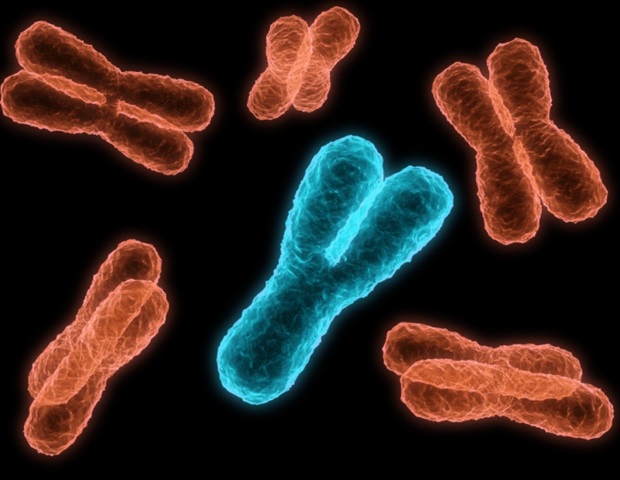
Researchers from Tokyo Metropolitan University have taken a big step in solving the mystery around why animals evolve sex chromosomes. It had long been proposed that sex chromosomes evolve to reduce “sexual conflict,” the evolution of features which are sub-optimal for either sex. By using fruit flies, the team showed that genes on newly formed neo-sex chromosomes in fruit flies tend to evolve “sex-biased genes” which give sex-specific phenotypes.
Chromosomes are neatly packaged bundles of DNA that carry all the genetic material of an organism. While prokaryotes (e.g. bacteria and archaea) typically have only one, more complex organisms tend to have many. Humans, for example, have forty-six. Out of these chromosomes, a subset known as sex chromosomes are known to determine the sex of individual animals. However, the evolution of sex chromosomes has continued to pose a puzzle for evolutionary biologists. The human Y chromosome, for example, is losing genes over time; it is estimated that it might be lost in several million years. This begs the question of why sex chromosomes evolved in the first place.
One potential answer to this question is in the reduction of what biologists call “sexual conflict.” When certain phenotypes or features (e.g. different body size) are beneficial to a specific sex but harmful for the other, a common phenotype for both sexes would lead to non-optimal results for everyone. The evolution of sex chromosomes might solve this conundrum by imparting certain phenotypes to a certain sex. However, as convincing as this sounds, it is difficult to prove. That is because sex chromosomes are normally very old; having evolved such a long time ago, all kinds of other effects from the environment will have contributed to genetic evolution in the meantime.
To get around this challenge, Anika Minovic and Associate Professor Masafumi Nozawa from Tokyo Metropolitan University have turned to Drosophila fruit flies, specifically ones with relatively recently evolved sex chromosomes, so-called neo-sex chromosomes. By comparing the species with related species which do not have one, they looked to whether the newly obtained sex chromosome led to the acquisition of “sex-biased genes,” that is, genes which impart phenotypes beneficial to either sex.
Comparing how genes on different chromosomes evolved, they found that many genes on neo-sex chromosomes tended to evolve into sex-biased genes, particularly at the larval stage. This is unexpected, since larvae tend not to have pronounced sex-specific features (sexual dimorphism). Such features can impact differences as an adult though. When we think about sex-dependent size, adult insects cannot grow much further due to a hard exoskeleton, so any beneficial contrast in size between sexes needs to be locked in at the larval stage. This corresponded precisely with the team’s findings that the sex-biased genes were, in fact, associated with metabolism, which would directly impact their size and reduce the sexual conflict inherent in a common body size.
This strongly supports the hypothesis that sex chromosomes evolve to reduce sexual conflict. The team is now continuing to pursue more direct measures for sexual conflict which might shed further light on this important question for evolutionary biology.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 17H05015, 15K14585, 21H02539, 25711023, 16H06279, and 221S0002, and by Tokyo Metropolitan University.
Source:
Tokyo Metropolitan University
Journal reference:
Minovic, A., & Nozawa, M. (2024). Evolution of sex‐biased genes in Drosophila species with neo‐sex chromosomes: Potential contribution to reducing the sexual conflict. Ecology and Evolution. doi.org/10.1002/ece3.11701.