Zika virus (ZIKV) infection is a potentially teratogenic illness, causing up to one in seven infants born to infected mothers to suffer neurodevelopmental deficits. A new study published in the journal Cell shows that some mothers escape injury to their babies because they develop a highly potent neutralizing antibody. This shows the importance of such antibodies in protecting the fetus against the Zika virus while presenting a possibly useful antibody for preventing fetal infection.
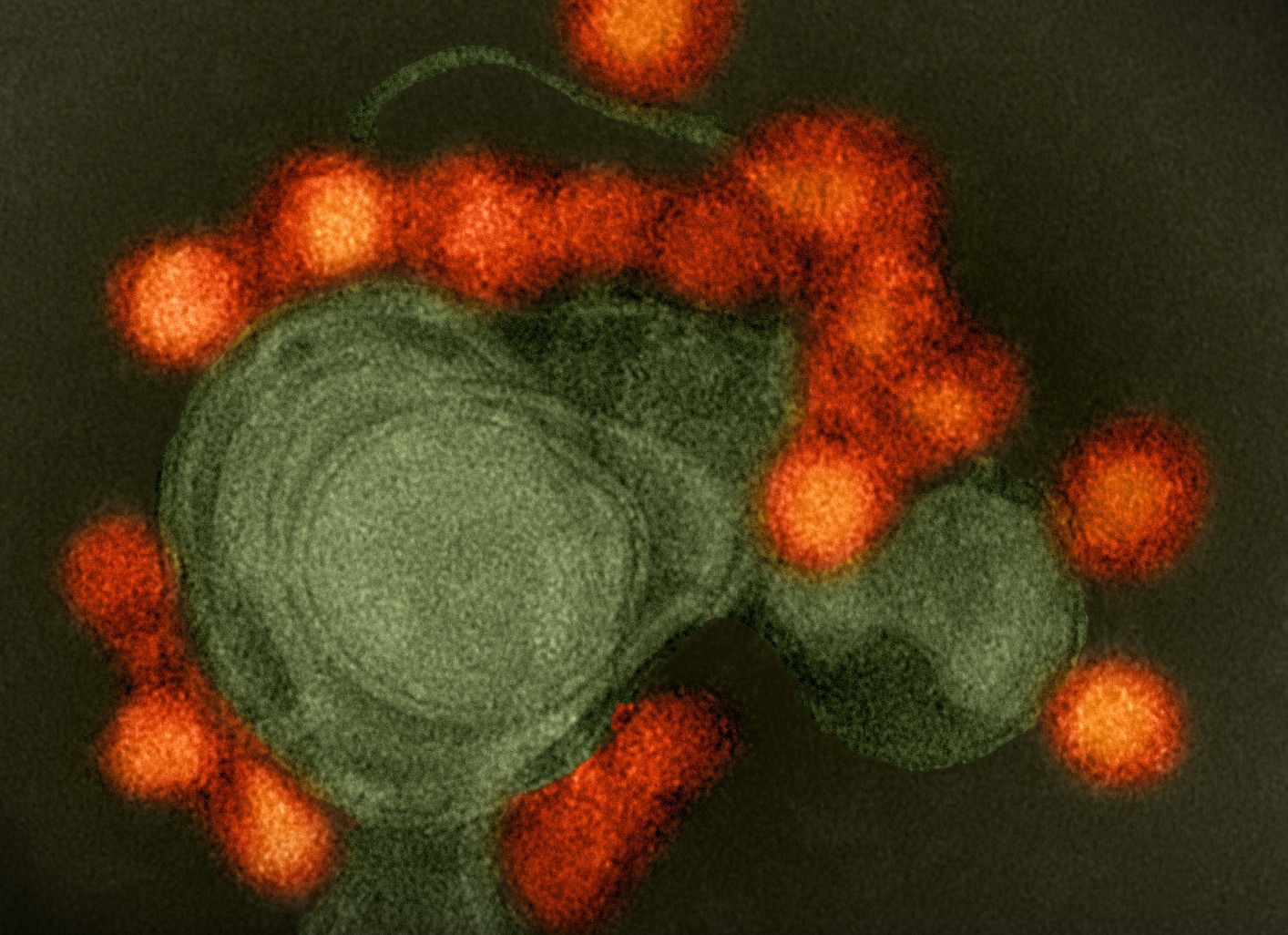
 Study: A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization. Image Credit: NIAID
Study: A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization. Image Credit: NIAID
Introduction
ZIKV emerged in the Americas and soon proved to be vertically transmitted, causing a significant minority of affected infants to be born with microcephaly and other congenital anomalies. Meanwhile, there was a significant rate of neurodevelopmental defects even among normal-appearing infants following maternal ZIKV infection during the pregnancy, some years before.
Interestingly, ZIKV does not typically cause adult diseases; it is primarily responsible for teratogenic outcomes during pregnancy. For instance, there were 11,000 cases of microcephaly in Brazil, originating from a single outbreak, during 2015-16. Therefore, protective immunity is needed during pregnancy, involving neutralizing antibodies (nAbs).
Earlier studies have primarily reported nAbs of the immunoglobulin G (IgG) class, even though IgM antibodies play a surprisingly prolonged role in flavivirus infections such as yellow fever virus and West Nile virus infections. This is based on the conventional concept of IgM antibodies being formed transiently during the acute phase of infection, comprising low-affinity antibodies lacking neutralizing capacity. However, this belies the early neutralizing IgM antibodies in such infections.
IgM antibodies are pentameric and bind to five times as many epitopes as IgG antibodies. This takes advantage of densely packed repeating structures on these viruses, allowing B cell receptors to be bound by the IgM antibodies in multivalent ratios. This, in turn, favors B cell stimulation and clonal selection of antibodies.
The outcome is specific IgM antibodies to ZIKV after several years, in contrast to the five-day half-life of IgM in the ordinary case. This indicates the selection of B cells expressing ZIKV-specific IgM, followed by expansion, during such infection.
“Though neutralizing activity is primarily attributed to IgG isotype antibodies, IgM may have an underappreciated role in ZIKV immunity… especially in pregnancy.”
B cells are first stimulated to release interleukin (IL-10), then their production is suppressed, allowing mature B cells to survive but reducing naïve B cell numbers during pregnancy. Since the latter produce IgM antibodies, this shift to memory and antibody-secreting cells shapes the profile of IgG and IgM nAbs during pregnancy.
The current study, to be published in Cell, explored the neutralizing capacity of IgM antibodies in pregnant women. The cohort comprised ten women, eight with acute and the others with secondary ZIKV infection, i.e., following previous dengue virus (DENV) exposure.
What did the study show?
The results show that all infected women showed high levels of anti-ZIKV antibodies throughout pregnancy from the time of infection. Anti-ZIKV IgM was found in all subjects, with one subject showing persistent IgM at 406 days
The researchers also found that ZIKV is partly neutralized by IgM antibodies, with the highest percentage being found at ~80% in one subject, who also showed the most prolonged period of persistence of ZIKV-neutralizing IgM at 100 days from symptom onset. Thus, neutralizing IgM is most important within the first trimester of pregnancy, with primary or secondary ZIKV infection.
However, four of the seven women with late IgM antibodies showed neutralizing capacity at up to ~210 days from symptom onset, all being secondary cases. Interestingly, the bulk of ZIKV-reactive IgM was not made up of ZIKV-neutralizing IgM.
The researchers looked at B cell repertoires from the blood samples taken from infected mothers.
This led to the identification of nine separate B cell lines that produced ZIKV-binding antibodies in the peripheral blood of ZIKV-infected mothers. In addition, these B lymphoblastoid cell lines (B-LCL) were found to include one that produced the IgM antibody DH1017.IgM, in pentameric form.
This nAb had matured, as shown by the fact that it had undergone somatic mutation. It was highly specific to ZIKV, being non-reactive to other flaviviruses, and showed ultrapotent neutralization of ZIKV. When directly compared to well-known ZIKV-neutralizing IgG monoclonal antibodies (mAbs), its range of neutralization exceeded that of the latter by eightfold to over 10,000-fold. Any neutralizing antibody with a half-maximal inhibitory concentration (IC50) of less than 10 ng/mL may be termed ultrapotent.
This ultrapotency was directly related to its IgM isotype and recombinant DH1017. IgG antibodies showed much weaker interactions with ZIKV. Notably DH1017.IgM did not show antibody-dependent enhancement (ADE) of infection in vitro, nor did the B cell clone react with human autoantigens to cause autoimmune disease.
When given to mice before exposing them to lethal doses of the virus, it prevented viremia better than IgG antibodies. While all exposed control mice died of the infection, those treated with DH1017.IgM survived and showed a reduction in viremia to the limit of detection. Even at half-dosage, all animals survived, but the viremia was less effectively prevented. The antibody was detectable for up to four days from virus exposure.
Here again, “the decavalent DH1017.IgM pentamer protects against ZIKV disease in mice and controls viremia more efficiently than the bivalent DH1017.IgG monomer.”
These results show that the multivalent isotype of IgM is responsible for the superior neutralization capacity.
The virus has a five-fold symmetry, with multiple ectodomain (E) glycoprotein units on the surface. Further study on the antibody structure revealed the “arms of the DH1017.IgM pentamer can bend toward the surface of the virus and that each arm can contact the epitopes of neighboring asymmetric units.” This decavalent mode of antigen recognition gives the IgM antibody an edge over the bivalent contact possible with IgG antibodies.
IgM antibodies can also bind to pairs of epitopes on different virions, cross-linking them to form aggregates. Both modes of antigen recognition may act simultaneously.
What are the implications?
Based on mouse findings, DH1017.IgM looks like a good candidate for immunotherapy against ZIKV. Like other flaviviruses, ZIKV induces persistent IgM antibodies that could help neutralize the virus within the first three months of pregnancy.
This is supported by the prior observation that in most cases ZIKV infection is cleared within two weeks of infection, probably because of the early peak production of IgM antibodies that control the virus. The current study indicates that such is the case with pregnancy as well.
Future studies should investigate how IgM levels are related to persistent viremia. In the woman who had the most prolonged period of viremia, beyond the first peak of IgM neutralizing activity at 14 days from symptom onset, a second peak occurred at ~70 days, during which time DH1017.IgM appeared. The somatic mutation of this cell line suggests it is a memory B cell expressing IgM, perhaps from a pool of IgM+ memory B cells and plasma cells that can give rise to isotype-specific neutralizing IgM antibodies.
The mode of neutralization could be via binding to a quaternary epitope not previously known to be recognized by a potently neutralizing antibody. However, this type of epitope does mark one class of potently neutralizing IgG mAbs. Its use by this IgM antibody could prevent a fusogenic change of conformation of the E protein, inhibiting infection.
The ultrapotency of DH1017.IgM is mainly due to its multivalent binding, suggesting IgM antibodies could occupy “a functional niche that is exclusive to IgM in the context of pathogens with repetitive proximal structures.” Moreover, the activity of DH1017.IgM is enhanced in the presence of complement, reducing the risk of ADE still further.
“Importantly, DH1017.IgM-mediated protection against lethal ZIKV challenge in mice recapitulates protection and viral control conferred by potent IgG neutralizing antibodies.”
In the current scenario, with no ongoing ZIKV vaccine trials because of the relative rarity of this infection, pregnancy-safe interventions are urgently required to reduce the risk of congenital ZIKV. DH1017.IgM may be one measure to fill this gap, especially since IgM is not transferred across the placenta, unlike IgG, reducing the risk of fetal injury or ADE in infancy.